Acidification
… measurements made over the last few decades have demonstrated that marine CO2 levels have risen, leading to an increase in acidity. Ocean acidification is projected to adversely affect a number of valuable marine ecosystem services by making it more difficult for many organisms to form shells and skeletons.13 Some shellfish are highly vulnerable to ocean acidification and any impacts to these species are expected to negatively affect the economy. (Climate Action Benefits: Shellfish, EPA)
Shellfish shells are less likely to form in an acidic environment, which compromises the future of this valuable high-protein, low-fat food source. The evidence of ocean acidification in the Pacific Northwest is compelling. (Shellfish and Climate Change, Washington state Dept of Health)
Ocean acidification is already impacting many ocean species, especially organisms like oysters and corals that make hard shells and skeletons by combining calcium and carbonate from seawater. However, as ocean acidification increases, available carbonate ions (CO32-) bond with excess hydrogen, resulting in fewer carbonate ions available for calcifying organisms to build and maintain their shells, skeletons, and other calcium carbonate structures. If the pH gets too low, shells and skeletons can even begin to dissolve. (Ocean Acidification, NOAA)
The world’s shellfish are under threat as our oceans become more acidic. (The Conversation)
“Acidification,” the reduction of the pH of the ocean due to absorbing CO2, sounds scary — acids are corrosive, so turning the ocean acid is obviously bad for the things living in it. As the quotes show, it is often claimed as a serious risk due to the increased CO2 concentration responsible for climate change.
The problem with the term “acidification” and the references to an acidic environment is that the ocean is basic, pH greater than 7. Lowering the pH of a base neutralizes it, makes it less basic. Only when pH gets below 7, which would take about six hundred years at the present rate of decline, does the water become acidic; distilled water, pH 7, is not an acid. “Neutralization” sounds less scary than acidification but is a more accurate description of the process.
That fact alone shows that the image of the ocean becoming acid is wrong and that people who use it should not be trusted, are either scientifically ignorant or trying to exploit the ignorance of their audience. It does not show that the underlying argument is wrong. Organisms that live in the ocean are adapted to its present conditions, including its present pH, so might function less well if it became less basic. That is a legitimate argument even if presented with dishonest rhetoric.
There are, however, several reasons to doubt that the effect on oceanic organisms of the reduction that has occurred, or will in the foreseeable future, is large and negative.
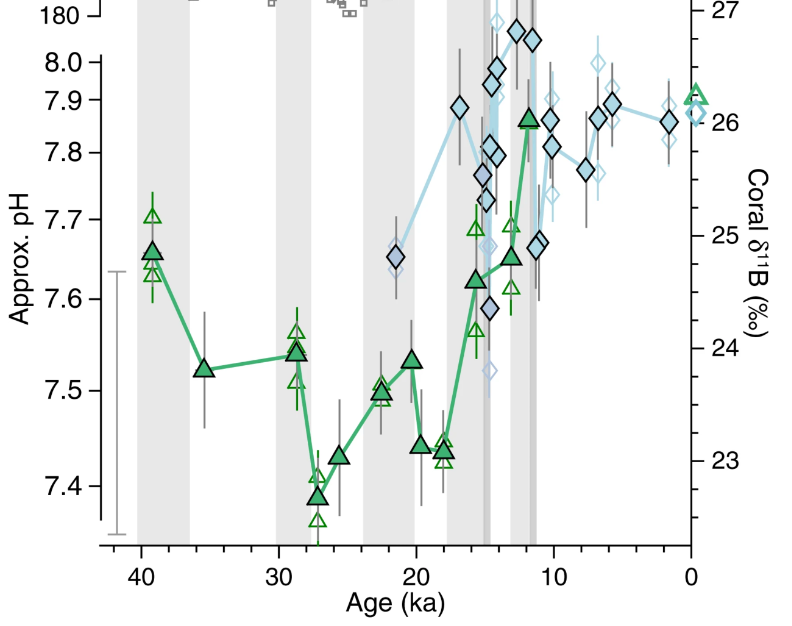
The first is that pH has varied considerably more in the recent past than it is varying at present, although probably not as rapidly. According to Extended Data Fig 1 in Rae et al., 2018, shown below, pH calculated from boron isotope proxy measurements was about 7.4 nineteen thousand years ago. It is currently 8.04 and falling by about .017/decade. At that rate it will take almost four hundred years for pH to get down to what it was nineteen thousand years ago.
Figure 1: (Rae et al., 2018, Extended Data Figure 1).1
One form of the argument, seen in quotes above, is the claim that “Ocean acidification is already impacting many ocean species, especially organisms like oysters and corals that make hard shells and skeletons by combining calcium and carbonate from seawater.” If that is correct, one would expect both current evidence in support and evidence from past variations in pH.
Coral: The Great Barrier Reef
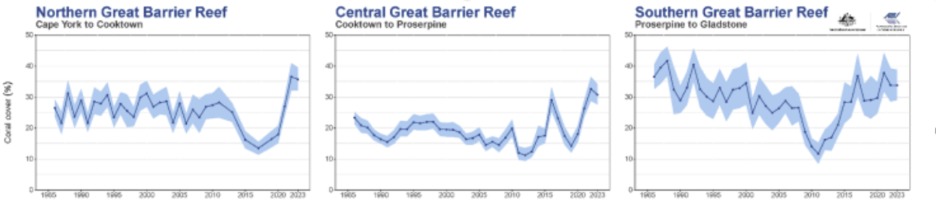
The claim that current levels of pH and temperature are affecting coral reefs is often supported with the case of the Great Barrier Reef. Current data from the Australian Institute of Marine Science, however, shows that its decline was temporary.
Figure 2: Hard coral cover of three regions of the Great Barrier Reef 1985 to 2023, AIMS 2023.
Further:
The Great Barrier Reef Marine Park Authority (GBRMPA) considers the earliest evidence of complete reef structures to have been 600,000 years ago. According to the GBRMPA, the current, living reef structure is believed to have begun growing on the older platform about 9,000 years ago. (Wikipeda, Great Barrier Reef)
Nine thousand years ago ocean pH was substantially lower than it is at present (Figure 1). If the current living reef structure began growing when ocean pH was 7.76 it is hard to see how it could have been destroyed by pH falling to 8.04 due to the effect of human production of CO2.
Oysters
Ocean acidification is already impacting many ocean species, especially organisms like oysters and corals that make hard shells and skeletons by combining calcium and carbonate from seawater.
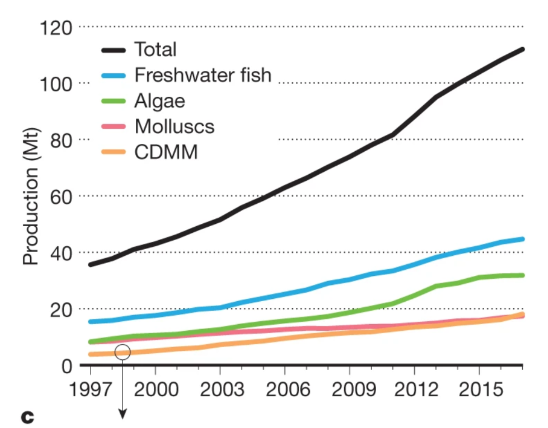
So far as what is already happening, one would expect the effect to have shown up in world production of mollusks, which has increased substantially over a period when ocean pH was declining, as shown on Figure 3.
Figure 3 from A 20-year retrospective review of global aquaculture, Nature 24 March 2021.
That does not prove that the claim is false, since a mild decline due to declining pH might be outweighed due to an increase from other causes, but it is at least evidence against and I have not yet found evidence in the other direction — readers are invited to provide any they are aware of.
What can we learn from fossil evidence? Oysters originated in the early Triassic, after the PETM extinction event:
we analysed well-preserved fossilised oysters (Liostrea hisingeri; see SI/Methods) collected from Lavernock Point, Wales, for their boron isotope composition (δ11B), which is a proxy for ocean pH. …
…With these assumptions, we calculate a minimum pH change of −0.29 units at 95 % confidence, from a pHi of 8.24 to a nadir of 7.93. (Pulses of ocean acidification at the Triassic–Jurassic boundary)
It is clear from the article that calculation of ocean pH in the deep past is difficult and uncertain, but it looks as though Oysters originated at a time when pH was unusually low.
[I was wrong about this, confusing the TJME with the later PETM, both of which involved periods of low pH. The TJME was at the end of the Triassic, so some fifty million years after Oysters appeared. I have not yet rewritten the argument, which may not work at all.]
My conclusion is that while it is possible that reduction in the pH of the ocean due to increased concentration of CO2 will have significant negative effects in the future there is less reason to believe it than frequently claimed and there is no evidence that it has had negative effects so far.
[I hope eventually to convert this post into a chapter in a book on consequences of climate change, so would be interested in comments by readers, in particular by readers who disagree with my conclusion. This particular climate issue is one I have started seriously looking into only recently.]
My web page, with the full text of multiple books and articles and much else
Past posts, sorted by topic
A search bar for past posts and much of my other writing
I have cut off the top part of the figure which shows atmospheric CO2 concentration over time.

Kudos for looking these things up. It is something everyone would benefit from, for the sake of amplifying the right ideas and dampening down the bad ones.
People are forwarding information from each other and then citing the sheer volume of discussion as if that is evidence. It reminds me a lot of a bad sound system for a stage production, where a mic picks up a speaker, sends a signal to an amplifier, which then drives that same speaker. The resulting feedback can be absolutely piercing.
The sound came from somewhere, originally, but the only parts you hear are the ones that hit the strongest positive feedback loops in the signal chain. It is often one single frequency, close to a sine wave, even though the original audio on stage has a broad mix of frequencies.
The way out is to add some filtering rather than just amplifying everything that comes by.
Evolution can be very fast as long as you're adjusting existing mechanisms rather than needing to make new ones. 10,000 years is plenty to adjust to slight pH changes. Equally it wouldn't surprise me if sea creatures could evolve fast enough to accommodate the current pH changes. But it's still a risk. They're likely happiest at whatever the recent levels are.
It shouldn't be that hard to grow some shelled molluscs in two tanks, one of which is slightly more acidic than the other, and see what happens?